| Chemistry 211-Exam 1 | Name: |
| September 19, 1997 | please print |
Answers:
1. d |
7. c |
13. d |
2. b |
8. d |
14. c |
3. d |
9. d |
15. c |
4. b |
10. a |
16. b |
5 .c |
11. a |
17. a |
6. a |
12. c |
18. c |
Solutions:
Instructions:
Read the whole examination before starting to work on individual problems. Some problems are easier than others; work those first. Budget your time to insure that you finish the examination. Don’t waste time on problems that you find hard to finish until you have worked the others.1. Which of the following is an example of a chemical change?
a. dry ice subliming |
c. sodium chloride melting |
b. alcohol boiling |
d. steel rusting |
Solution: The only answer where a substance is changing chemical composition is steel rusting.
2. The number of protons in the nucleus determines the
a. atomic mass |
c. mass number |
b. atomic number |
d. number of neutrons |
Solution: The number of protons tells us the identity of the element and is equal to the atomic number.
3. The formulae of the hydroxide, the nitrite, and the sulfite ions are represented respectively as
a. |
c. |
b. |
d.
- |
Solution: Recall the table that you needed to memorize! The correct
answer is ![]() .
.
4. The formula for aluminum carbonate is Al2(CO3)3. On the basis of this information, the formula for the phosphate of aluminum would be expected to be
a. Al2P3 |
c. Al(PO4)2 |
b. AlPO4 |
d. Al2(PO4)3 |
Solution: the formula tells you that Al has a +3 charge. Phosphate also has a +3 charge so that the formula that would give a neutral molecule is AlPO4.
5. The net ionic equation for the reaction of Mg(OH)2(s) with HCl is (recall which are strong and weak acids and bases)..
a. H+(aq) + OH- (aq)
® H2O(l)b. 2HCl(aq) + Mg2+(aq) + 2OH- (aq)
® MgCl2(s) + 2H2O(l)c. 2H+ (aq) + 2Cl- (aq) + Mg(OH)2(s)
® MgCl2(s) + 2H2O(l).d. 2Cl- (aq) + Mg2+(aq)
® MgCl2(s)Solution: HCl is a strong acid. This makes it ionic. Both H+ (aq) and Cl- are ionic. and are involed in the reaction. They form water with the magnesium hydroxide and the chloride of magnesium (which should have been soluble).
6. Name the following compound: N2O3.
a. dinitrogen trioxide |
c. nitrogen tetroxide |
b. dinitrogen tetroxide |
d. nitrogen trioxide |
Solution: Dinitrogen trioxide.
7. The formula of the perclorate anion is
a. HClO3 |
c. |
b. |
d. |
Solution: Use table! Per- tells us there is a lot of oxygen.
The correct answer is ![]() .
.
8. The molecular formula of a compound is (NH4)2C2O4.· H2O. Its molecular mass is
a. 92 amu |
c. 124 amu |
b. 106 amu |
d. 142 amu |
Solution: Sum the masses of all elements present in the molecule .![]()
9. When 1.68 g of benzene was burned, 5.68 g of CO2 was produced. Determine the mass of benzene burned if 15.0 g CO2 were produced.
a. 0.379 g |
c. 6.19 g |
b. 0.636 g |
d. 4.44 g |
Solution: Use law of definite proportions. Let mB = mass of benzene.
![]()
10. The mass of an atom X is 1.54x10- 22g; the atomic number of this element is
a. 41 |
c. 7 |
b. 25 |
d. 81 |
Solution: 1 mol of X is avAvagadro’s # of atoms. Thus
FM=1.54x10- 22g· 6.02x1023 = 92.6; This is the mass of element number 41.
11. Convert 3.44 kPa· dm3 to J if 1 J = 1 kg· m2/s2 and 1 Pa = 1kg/(m· s2)
a. 3.44 |
c. 3.44x103 |
b. 3.44x10- 3 |
d. 3.44x10- 9 |
Solution: Hopefully you recognize this as a unit conversion problem. Use definitions provided.
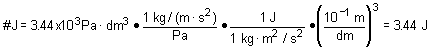
12. Naturally occurring boron consists of two isotopes, 10B and 11B with atomic masses 10.013 and 11.009 respectively. The observed atomic mass of boron is 10.811. Calculate the abundance of 10B.
a. 0.199% |
c. 19.9% |
b. 80.1% |
d. 0.801% |
Solution: remember that the atomic mass on the periodic table is the weighted average of the isotopic masses. Set up an equation for this.
![]()
13. If y = (3.15x10- 23)1/24, then y =
a. 0.347 |
c. 63.3 |
b. 1.31x10- 24 |
d. 0.115 |
Solution: Use your calculator to obtain 0.115
14. In chapter 5 we will discuss that pressure, P, and volume, V, are inversely proportional to each other according to the equation: PV = nRT. Determine the final pressure (in atm) of a cylinder if the initial pressure was 1.00 atm and the volume slowly decreased to exactly 1/3 of its initial volume. Assume all other conditions were constant.
a. 0.333 |
c. 3.00 |
b.1.00 |
d. not enough information given |
Solution: This should be recognized as a math skills problem.
Set up two simultaneous equations: |
|
Combine and solve for P2:: |
|
From what is given: |
|
Substitute: |
|
15. Determine the volume of concentrated solution required to produce 500.0 mL of 1.00 M NaCl from 3.00 M NaCl.
a. 0.500 L |
c. 0.167 dm3 |
b. 1500 mL |
d. 1.500 dm3 |
Solution:This is a dilution problem and then you need to do some unit analysis to determine which answer is correct.
Dilution |
|
Units |
|
16. Given that y = ln x - 2.303log 2x
a. 0.500 |
c. -0.301 |
b. -0.693 |
d. not enough information given |
Solution: This is a math skills problem.
Skill 1 |
ln 2x = 2.303· log 2x |
Skill 2 |
|
Asnwer |
- 0.693 |
17. When the equation
2 C5H6NS(l) + 17O2(g)
® 10 CO2(g) + 6 H2O(l) + 2NO2(g) + 2SO2(g)is balanced, the sum of the coefficients (simplest whole number) is
a. 39 |
c. 29 |
b. 33 |
d. 28 |
Solution: Sum is 39.
18. Which of the following is a strong acid?
a. HF |
c. HCl |
b. HClO |
d. HCNO |
Solution: From table in book HCl is a strong acid.
