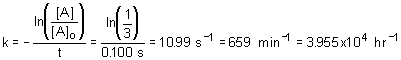| Chemistry 212 - Examination I | Name: |
July 9, 1997 |
please print |
Instructions: Read the whole examination prior to starting to work on individual problems. Work the problems that you find easiest first. Budget your time!
Answers:
1. b |
6. b |
11. c |
16. c |
2. c |
7. a |
12. a |
|
3. c |
8. a |
13. c |
|
4. a |
9. d |
14. a |
|
5. c |
10. b |
15. d |
Solutions:
1. Using the information below, we would predict that an increase in the temperature of a solution containing CaCl2 would cause ___.
![]()
a. the solubility to increase. |
c. D H to become positive. |
b. the solubility to decrease |
d. D H to become zero. |
Solution:
Determine whether the reaction for the dissolution of CaCl2 is exo- or endo- thermic using the thermochemical data; sum reactions as written to obtain
![]()
Negative sign means that solubility decreases with increases in temperature.
2. If CaCl2 were completely ionic, the van't Hoff factor, i, would be
a. 1 |
c. 3 |
b. 2 |
d. 4 |
Solution:
When 1 forumula unit of calcium chloride dissolves, it forms a Ca2+, and 2Cl
- ions. Thus three particles are formed in solution for every calcium chloride dissolved. i = 3.3.For a two-component system, one can calculate the mole fraction of the solvent directly, given only
a. the density of the solution. |
c. the mole fraction of the solute. |
| b. the molarity of the solution. | d. the molecular weight of the solvent. |
Solution:
With a two component system knowledge of mole fraction of one of the components means you can determine the mole fraction of the other by simply subtracting from 1. Thus mole fraction of solvent would be obtained from mole fraction of solute.
4. Which of the following increases the solubility of a gas in a given solvent?
a. increasing the partial pressure of the gas. |
c. increasing the temperature of the solvent. |
b. decreasing the partial pressure of the gas |
d. both a and c |
Solution:
Increase pressure of gas.
5. The initial rate of a chemical reaction
- is always constant at any temperature.
- is independent of the amount of catalyst.
- is approximately doubled for a 10°C rise in temperature.
- varies inversely with the absolute temperature.
Solution:
Rule of thumb: an approximate increase of a factor of 2 in the rate is observed with a 10°C increase in temperature.
6. The detailed sequence of elementary steps that lead to the net overall reaction is called the reaction
a. potential |
c. rate law |
b. mechanism |
d. stoichiometry |
Solution:
Mechanism!
7. An aqueous solution of HCl had a concentration of 0.130 m. The mole fraction of HCl was
a. 2.33x10-3 |
c. 0.130 |
b. 1.0 |
d. 0.870 |
Strategy:
1. Molality is defined as mol solute per kg of solvent. For 1 kg of solvent we would have 0.130 mol os solute.
2. Mole fraction solute is defined as mol of solute per total number of moles of solution.
3. Use 0.130 mol solute and convert 1 kg of solvent to moles of solvent.
Solution:
| Mole of solvent(water): | |
| Mole fraction: |
8. The freezing point of a 0.700 molal solution was
-5.208° C (Kf = 1.86° C/molal), the van't Hoff factor, i, must bea. 4 |
c. 2 |
b. 3 |
d. 1 |
Solution:
Use the freezing point depression equation :
DT = 5.208°C; molality = 0.700 m; Kf = 1.86° C/molal![]()
9. At body temperature of 37
° C, what is the osmotic pressure of a physiological saline solution, 0.90% by weight NaCl, in water? The density is 1.00 g/mL at 37° C.a. 16 atm |
c. 0.15 atm |
b. 1.4 atm |
d. 7.8 atm |
Strategy:
Use the equation for osmotic pressure (
p = MiRT); convert mass % to molarity; don’t forget to include i = 2 to account for the fact that NaCl is ionic.Solution:
| 1. mass % to molarity | |
| 2. Osmotic pressure | p = 0.154 MÄ 2Ä 0.08206Latm/molKÄ 310K = 7.84atm |
10. Henry's law constant for O2 is 7.8x102 atm
× M- 1. Assume that the partial pressure of O2 is 0.20 atm. Determine the mole fraction of O2 in water. Watch your units!!! Assume a density of 1.00 g/mL for the solution.a. 2.56x10- 4 |
c. 6.44x10 - 3 |
b. 4.61x10 - 6 |
d. 1.15x10 - 4 |
Strategy:
Remember that solubility of a gas is proportional to the pressure of the substance above the solution. We can also express this as pressure above the solution is proportional to the solubility in the solution. We use the units to guide us! P = k
Ä S ; after determining solubility convert to mole fraction.Solution:
| Solubility: | |
| Mole fraction | 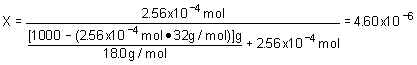 |
11. Given that the vapor pressure of water at 75
° C is 290 mmHg; the vapor pressure of an aqueous solution containing 180 g of water and some urea (molecular weight = 60.0 g/mol) at 75° C is 264 mmHg. The mass of urea dissolved in 180 g of water isa. 0.90 g |
c. 60 g |
b. 0.10 g |
d. 161 g |
Strategy:
This is a problem where Raoult’s law must be used to determine the mole fraction of solute in the solution. Knowing the moles of water in the solution you can determine the moles of urea that must be present. From the moles of urea and molecular weight you can determine the mass of urea.
Solution:
| Mole of solute from mole fraction.. |  |
| Mass from moles | m = 0.98Ä 60 » 60 g |
12. What is the first order rate constant for the beta decay of Tc if the half-life is 2.2x105 years?
| a. 1.0x10- 13/sec. | c. 1.2x10- 8/day. |
| b. 4.5x10- 6/year. | d. 5.2x10- 10/hr. |
Solution:
Use relationship between first order rate constant and half-life to determine constant; rest is unit conversion problem (Chapter 1).
![]()
13. For the reaction (CH3)3CCl + OH
- « (CH3)3COH + Cl- it is experimentally found that doubling the concentration of (CH3)3CCl causes the reaction rate to increase by twofold, but doubling the concentration of OH- has no effect on the rate. The rate equation isa. R = k[(CH3)3CCl][OH- ] |
c. R = k[(CH3)3CCl] |
b. R = k[(CH3)3CCl]2[OH - ] |
d. R = k[(CH3)3CCl][Cl - ] |
Solution:
When doubling the concentration,doubles the rate the order for that substance is 1; when there is no change in the rate with changes in the amount of a compound, the only possible order would be zero (since anything raised to the zero power is 1). Thus the rate law is expressed as R = k[(CH3)3CCl].
14. The rate constant for a reaction is 1.3M
- 1s- 1 at 700K and 23M- 1s- 1 at 800K. What is the activation energy? R = 8.31 Jmol- 1K- 1.a. 134 kJ/mol |
c. 17.7 kJ/mol |
b. 34.7 kJ/mol |
d. 4.26x10 - 3 J/mol |
Solution:
Simply use the Arrhenius equation solving for the activation energy.
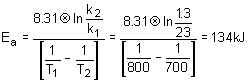
15. The reaction CHCl3(g) + Cl2(g)
« CCl4(g) + HCl(g) has been proposed to occur by the mechanism1 |
Cl2 ® 2Cl |
fast |
2 |
2Cl ® Cl2 |
fast |
3 |
CHCl3 + Cl ® HCl + CCl3 |
slow |
4 |
CCl3 + Cl ® CCl4 |
fast |
The overall rate law, which is consistent with this mechanism, is
a. |
c. |
| b. |
d.  |
Solution:
Use the rate limiting step to determine the rate law; eliminate any intermediates using the steady state approximation.
| 1. Rate law: | |
| 2. Steady state approximation: | 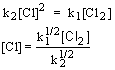 |
| 3. Substitute: | 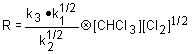 |
16. For a first order reaction, the concentration of reactant decreased to exactly 1/3 of its initial value in 100 ms, the rate constant for this reaction is
a. - 10.99 s- 1 |
c. 39550 hr - 1 |
b. 4.77 s - 1 |
d. 0.183 min - 1 |
Solution:
Use integrated first order rate equation, solving for rate constant; use unit analysis to determine the correct answer.