| Chemistry 212 - Examination 3 | Name: |
April 24, 1998 |
please print |
| 1. c | 6. a | 11. a |
| 2. a | 7. d | 12. d |
| 3. c | 8. a | 13. d |
| 4. b | 9. b | 14. b |
| 5. b | 10. c | 15. b |
Instructions: Read the whole examination prior to starting to work on individual problems. Work the problems that you find easiest first. Budget your time!
1. The solubility of PbF2(s) (g/L) can be increased by addition of
| a. NaOH | c. HNO3 |
| b. NH3 | d. H2O |
2. The free energy change of a phase change (at or near equilibrium) would be
| a. zero | c. positive |
| b. negative | d. equal to TD H - D S |
3. The change in enthalpy for the reaction below has a negative sign. Which statement below is correct?
2NH3(g) + CO2(g) ® NH2CONH2(aq) + H2O(l)
The reaction is
| a. spontaneous at all T. | c. spontaneous at low T but not high. |
| b. spontaneous at high T but not low. | d. never spontaneous |
4. Which statement below is correct for the reaction: 2Li+(aq) + 2F- (aq) ® F2(g) + 2Li(s)?
| a. the cell voltage is positive and the free energy is negative. | c. the cell voltage is positive and the free energy is positive. |
| b. the cell voltage is negative and the free energy is positive. | d. the cell voltage is negative and the free energy is negative. |
5. The anode in the cell, for which the reaction is Fe2+(aq)+ Zn2+(aq) ® Zn(s) + Fe3+(aq), could be:
| a. Fe2+. | c. Zn |
| b. Pt | d. Zn2+ |
6. AgCl was dropped into a solution containing 1.00 M NaCl and 1.00 M NaCN. Without doing any calculations estimate the approximate solubility. The solubility of AgCl in pure water is 1.34x10- 5 M. Given Kf = 5.6x1018 for Ag(CN)2- .
| a. >1.34x10- 5 M | c. <1.34x10- 5 M |
| b. = 1.34x10- 5 M | d. Unable to tell |
7. What is the solubility of PbI2(s) in 0.100 M NaI if the Ksp is 6.5x10- 9.
| a. 1.17x10- 3 M | c. 2.6x10- 6 M |
| b. 1.6x10- 8 M | d. 6.5x10- 7 M |
8. A solution of zinc (II) ions was saturated with H2S (0.100 M). Determine the equilibrium concentration of zinc ions at a pH of 2.50 if Ksp(ZnS) = 1.1x10- 21. Given
H2S(aq) « 2H+(aq) + S2- (aq) K = 1.1x10- 20
| a. 1.0x10- 5 M | c. 1.1x10- 20 M |
| b. 3.3x10- 11 M | d. 5.0x10- 6 M |
9. A compound exists as either a perfect crystal or as a crystal with four equivalent orientations. Determine the number of moles of the compound in the perfectly crystalline form in a solid that is a mixture of these two states if the total entropy of one mole of the compound at absolute zero is 5.18 J/K.
| a. 0.55 mol | c. 0.97 mol |
| b. 0.45 mol | d. 0.03 mol |
10. How many moles of cadmium metal will be deposited on an electrode if 1.33 A of electric current was passed through an aqueous solution of CdSO4 for 122 min? F = 96485 C/mol
| a. 8.40x10- 4 mol | c. 0.050 mol |
| b. 1.68x10- 3 mol | d. 0.101 mol |
11. To a solution containing 0.100 M NaCl and 0.100 M NaI was added 0.100 M Pb(NO3)2. Given: Ksp (PbI2) = 6.5x10- 9 and Ksp(PbCl2) = 1.6x10- 5. What is the [I- ] when Cl- starts to precipitate?
| a. 2.01x10- 3 M | c. 3.55x10- 4 M |
| b. 7.11x10- 4 M | d. 1.51x10- 3 M |
12. Determine the change in the internal energy during the reaction below at 25°C. Assume we can ignore the volume of liquids and solids.
H2(g) + ½O2(g) ® H2O(l) + 187.8 kJ
| a. - 186.6 kJ | c. - 189.0 kJ |
| b. - 191.5 kJ | d. - 184.1 kJ |
13. Determine D G for a weak acid having a Ka = 1.75x10- 6 at 25.0 °C if [HA ] = 0.150 M = [A- ] at pH = 3.33.
| a. - 6.01 kJ | c. - 13.8 kJ |
| b. +6.01 kJ | d. +13.8 kJ |
14. The potential of the cell below was 1.05 V. What is the pH? Eo(Ag) = 0.799 V
Pt| H2(1atm)| H+ (aq)|| Ag+(0.100 M)| Ag
| a. 10.50 | c. 1.00 |
| b. 5.25 | d. 7.00 |
15. Determine D G° of combustion at 25.0°C from the information below:
| C6H6 (l)+ | 7.5O2(g)« | 6CO2 (g)+ | 3H2O(l) |
![]()
| a. +3202 kJ | c. - 2935 kJ |
| b. - 3202 kJ | d. +2935 kJ |
Solutions:
1: Increase the solubility by decreasing the amount of product. Amount of [F- ] would decrease if acid, such as HNO3, was added.
2: At or near equilibrium means D G = 0!
3: D S is negative since there are more moles of gaseous reactions that products. With a negative entropy change - TD S will be positive and at high temperature will be greater than D H. At low temperature this term will be small and the reaction is enthalpy driven.
4: Li+ and F- are favored; D G should be positive and cell voltage is negative.
5: Anode is a solid substance at which oxidation occurs. Iron is oxidized from +2 to +3 state; a solid electrode of some other material is needed. Pt is usually used.
6: The presence of chloride ion decreases the solubility; the presence of cyanide increases the solubility and does so by a lot more (note the large Kf and squared dependence of cyanide concentration.
7: An example of the common ion effect.

8: This is just like the problem in class and in the book; use the pH to determine [S2- ]. Substitute into solubility equation and determine zinc concentration.
| 1. [S2- ] | 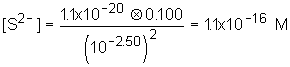 |
| 2. [Zn2+] | 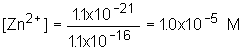 |
9: The part of the mixture that is in the perfect crystalline state does not add to the total entropy. This means that the total entropy is due to the other state; comparison of the entropy for one mole of that state to the entropy of one mole of the mixture gives the entropy of the imperfect crystalline form.
S = R ln 4 = 11.5 J/mol
![]()
10: In class we solved a problem much like this. Cadmium is in the +2 oxidation state in this compound; this means that n =2.
![]()
11: This is nearly the same problem we worked in class right before the exam! Determine the lead concentration when the chloride starts to precipitate; use this concentration in the other equilibrium to determine the molarity of iodide.
| 1. Lead concentration | 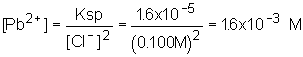 |
| 2. Iodide concentration | 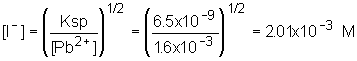 |
12: Determine the work and add it to the heat involved in the reaction.
D U = q - PD V = q - D nRT
![]()
13: Remember the relationship discussed in class for free energy change at
non-standard state conditions, i.e., ![]() . You are given T and K;
determine Q from given concentrations.
. You are given T and K;
determine Q from given concentrations.
![]()
\ 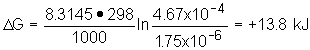
14: From what is given we should realize that hydrogen is being oxidized and silver
ions are being reduced. Since the hydrogen electrode is our reference point, we know that
the Eo for the cathode is zero. This gives ![]() . Use Nernst
equation to determine the hydrogen ion concentration and then convert to pH.
. Use Nernst
equation to determine the hydrogen ion concentration and then convert to pH.
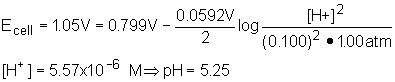
15: Determine DHo and DSo and from these DGo.
D Ho = [3(- 285.8) + 6(- 393.5)] - [7.5(0) + 1(49.0)] = - 3267.4 kJ
D So = {[3(69.94) + 6(213.7)] - [7.5(205.0) + 1(172.8)]}/1000 = - 0.218 kJ
D G = D Ho - TD So = - 3267.4 kJ - 298(0.218 kJ) = - 3202 kJ