EQUATIONS
![]()
![]()
![]()
M = n/V
CONSTANTS
Ek = 1/2mv2
NA = 6.02x1023/mol
q = nC
Dtc = 2.998x108 m/s
q = s
×m×Dth = 6.626x10- 34 J× s
l
= h/mvR = 0.0821 L
×atm/(mol×K)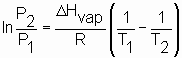
= 8.314 J/(K×mol)
![]() 0
0
e = 1.603x10- 19C
c =
lnR = 1.097x 10- 2 nm- 1
W = - PD V
1Å = 10- 10m
D
H = q p1 J = 1 kg
×m2×s- 2D
H = DE + D(PV)l
= 2dsinqPV = nRT
1 torr = 760 mm Hg = 1 atm
![]()
1 cal = 4.184 J
P1V1 = P2V2
1 W = 1 J/s
![]()
E = h
n![]()
XA = nA/n = PA/P
![]()
![]()
E = q + w
D G = D H - TD S