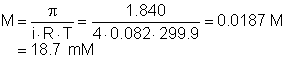|
Chemistry 212-Exam 1 |
Name: |
|
|
March 10, 1999 |
|
please print |
Answers:
1. b 7. d 13. a
2. b 8. b 14. c
3. c 9. d 15. b
4. d 10. c 16. a
5. c 11. d
6. a 12. b
Instructions: Read the whole examination before starting to work on individual problems. Work the easiest problems first. Budget your time to insure that you finish the examination. Don’t waste time on problems that you find hard to finish until you have worked the others. Remember the use of programmable calculators is forbidden and would be considered a serious honor code violation.
Solutions:
1. The rate constant is not a function of which of the following?
|
a. temperature |
c. fraction of collisions with sufficient energy |
|
b. the concentration of reactants |
d. orientation of the molecules |
Solution1: The rate is a function of the concentrations, but not the rate constant.
2. The vapor pressure of pure ethanol (ETOH) is greater than the vapor pressure of pure water. Assuming ideal behavior, upon addition of ethanol to water the vapor pressure (vp) of the solution should be
|
a. greater than the vp of pure ETOH |
c. the same |
|
b. greater than the vp of pure water |
d. less than the vp of pure water |
Solution2: the vapor pressure of the liquid (solution) will be between the vapor pressures of the two compounds.
3. For the reaction A « 2B Kc = 1.396, if one started with 0.184 M A approximately how much A & B would there be after equilibrium had been established?
|
a. 0.266 M A; 0.051 M B |
c. 0.051 M A, 0.266 M B |
|
b. 0.184 M A; very little B |
d. very little A, 0.184 M B |
Solution3: Kc is neither very small nor very large. There should be reasonable amounts of reactant and product; there is a bit more product than reactant in this reaction since the equilibrium constant is greater than one.
4. Which substance in the reaction below is an intermediate?
|
A + B |
« |
C + D |
|
|
|
D + E |
« |
F |
|
|
|
a. B |
c. A |
||||
|
b. C |
d. D |
||||
Solution4: Intermediates appear in one step and are consumed later- thus they do not appear in the net reaction!
5. What is the rate of consumption of A (M/s) if the rate of consumption of B is 0.386 M/s? Use the reaction below:
3A + 2B 4C « products
|
a. 0.386 M/s |
c. 0.579 M/s |
|
b. 0.289 M/s |
d. 0.257 M/s |
Solution5: Use the ration of the stoichiometric coefficients multiplied by the rate to determine the rate of A!
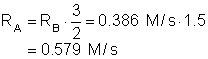
6. Determine the solubility of H2 at 20°C if the Henry’s law constant is 8.50x10-4M/atm and the pressure of H2 is 3.1511 atmospheres (M = added during exam.).
|
a. 2.6784 mM |
c. 2.3210 mM |
|
b. 5.3568 mM |
d. 0.05357 mM |
Solution6: Use Henry’s law!
![]()
7. The oxidation of nitric oxide to form nitrogen dioxide occurs according to the reaction below. Determine the equilibrium constant, Kc, if the equilibrium concentrations of NO, O2, and NO2 were 0.189 M, 0.272 M, and 0.388 M, respectively, at 25°C.
2NO(g) + O2(g) « 2NO2(g)
|
a. 0.13 |
c. 12.01 |
|
b. 0.0641 |
d. 15.60 |
Solution7: Use the standard relationship between equilibrium constant and equilibrium concentrations.
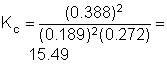
8. What is the Kp in the reaction below, if Kc = 7.86x10-5 at 234.9°C
3A(s) + 4B(g) « 3C(g)
|
a. 4.08x10-6 |
c. 3.28x10-3 |
|
b. 1.88x10-6 |
d. 2.60x10-11 |
Solution8: Use the standard relationship between Kp and Kc, but don’t include solids! Use R = 0.08206 and T = 234.9 + 273.15 = 508.05 K; Dn = -1
![]()
9. What is the fraction of A remaining after 54.178 minutes if the first order rate constant is 4.010x10-4 s-1?
|
a. 0.7284 |
c. 0.9503 |
|
b. 0.0497 |
d. 0.2716 |
Solution9: The fraction remaining is the ratio of the concentration at 54.178 minutes to the initial concentration; use the first order rate equation. Convert time to sections since that is the unit of the rate constant! Thus, t = 3251 s
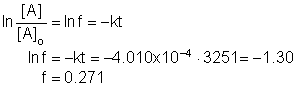
10. Initially there were equilibrium concentrations of 0.1572 M A and 0.1577 M B. Which one of the equations below is correct if the volume decreased by a factor of 1.80?
3A(g) « 5B(g)
|
a. Qc = 1.67Kc |
c. Qc = 3.23Kc |
|
b. Qc = 0.08Kc |
d. Qc = 0.31Kc |
Solution10: It doesn’t matter what the equilibrium concentrations were before the change. The decrease in volume causes an increase in the concentrations of each by the factor given.
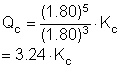
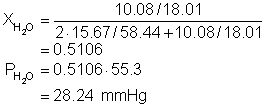
11. What is the vapor pressure of a solution at 40.0°C produced by dissolving 15.6796 g of NaCl (formula mass = 58.44 g/mol) with 10.0811 g of water? The vapor pressure of pure water at this temperature is 55.30 mm Hg. Assume that the solute is completely ionic.
|
a. 46.15 mmHg |
c. 27.07 mmHg |
|
b. 9.15 mmHg |
d. 28.23 mmHg |
Solution11: Determine the mole fraction of water and using Raoult’s law multiply by the vapor pressure!
Mole fraction of water:
12. Determine the mole fraction if LiI in a 13.034 wt% aqueous solution of LiI.
|
a. 0.0473 |
c. 0.0527 |
|
b. 0.0198 |
d. 0.0098 |
Solution12: Determine the moles of LiI and H2O in 100 g of solution.
|
mole LiI |
= 13.034/133.841 |
|
|
= 0.09735 mol |
|
mol H2O |
= (100.00 - 13.03)/18.01 |
|
|
= 4.829 mol H2O |
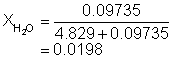
13. A mixture containing an initial concentration of 0.1483 M for N2 and 0.1168 M for O2 is allowed to come to equilibrium (see reaction below). What must be the equilibrium concentration of NO?
N2(g) + O2(g) « 2NO(g) Kc = 2.700x10-18
|
a. 2.16x10-10 |
c. 6.66x10-11 |
|
b. 5.40x10-11 |
d. 1.08x10-10 |
Solution13: Setup equilibrium equation and solve for the unknown concentration using the quadratic equation or approximate; note that the amount that reacts must be small since the equilibrium constant is small. This simplifies the calculation dramatically.
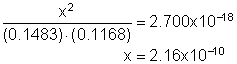
14. The following data were obtained by the method of initial rates. What is the rate law for the reaction of A with B?
|
[A]o |
[B]o |
Rate,M/s |
|
|
|
0.229 |
0.354 |
1.68x10-6 |
|
|
|
0.446 |
0.354 |
4.56x10-6 |
|
|
|
0.229 |
0.568 |
2.12x10-6 |
|
|
|
a. Rate = k[A]0[B]1 |
c. Rate = k[A]1.5[B]0.5 |
|||
|
b. Rate = k[A]1[B]0 |
d. Rate = k[A]0.5[B]1.5 |
|||
Solution14: Use sets of data to determine each coefficient. The first two gives the exponent for A and the first and third set give the coefficient for B. We may be able to stop after determining only one of them due to the choices given!
For A:
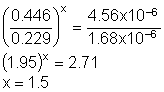
Note: only c is possible!
15. What is the freezing point depression when 16.53 g of ascorbic acid (formula mass = 176.06 g/mol) is dissolved in 186.60 g of diethyl ether? The freezing point depression constant is 1.790 °C*kg/mol
|
a. 17.523°C |
c. 1.061°C |
|
b. 0.901°C |
d. 0.089°C |
Solution15: Use the freezing point depression equation. After determining the molality of solute.
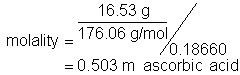
![]()
16. The osmotic pressure of a solution of Al(NO3)3 is 1.840 atm at 26.90°C. What is the analytical (not the particle) molarity of Al(NO3)3? Assume the Al(NO3)3 is completely ionic!
|
a. 18.681 mM |
c. 164.934 mM |
|
b. 21.208 mM |
d. 4.670 mM |
Solution16: Use the osmotic pressure equation. Since we are to assume the solution is completely ionic, we use i = 4.