|
Chemistry 212-Exam 2 |
Name: |
|
|
April 7, 2000 |
|
please print |
Answers:
|
1. a |
7. b |
13. c |
|
2. d |
8. b |
14. b |
|
3. a |
9. c |
15. a |
|
4. d |
10. c |
16. b |
|
5. c |
11. b |
|
|
6. a |
12. c |
|
Solutions:
Instructions: Read the whole examination before starting to work on individual problems. Work the easiest problems first. Budget your time to insure that you finish the examination. Don’t waste time on problems that you find hard to finish until you have worked the others. Remember the use of programmable calculators is forbidden and would be considered a serious honor code violation.
1. Which reactant in the reaction below is a base (this one first in the answer) and which reactant is an acid?
S2-(aq) + HBrO(aq) « HS-(aq) + BrO-(aq)
|
a. S2-, HBrO |
c. HBrO, BrO- |
|
b. S2-, BrO- |
d. HBrO, S2- |
Solution1: On the left-hand side, HBrO is giving up a proton and S2-is accepting it, making them an acid and base respectively.
2. What is the conjugate base and base dissociation constant of H2O2 if it’s acid dissociation constant is 2.400x10-12?
|
a. |
c. |
|
b. |
d. |
Solution2: Since H2O is acting as an acid, it’s conjugate base will have one less proton. Determine Kb by dividing Ka into Kw.
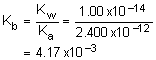
3. A solution with a hydronium ion concentration of 7.12x10-6 is ___ and the hydroxide concentration is ___.
|
a. acidic, 1.40x10‑9 M |
c. basic, 1.40x10‑9 |
|
b. basic, 1.40x10+5 |
d. acidic, 1.40x10+5 |
Solution3: Since the hydronium concentration is greater than 1.00x10-7 M, it is acidic. The hydroxide concentration will be low (it can be calculated from Kw = [H3O+][OH-]).
4. Which of the acids below has the weakest conjugate base?
|
a. CH3COOH |
c. Cl2CHCOOH |
|
b. ClCH2COOH |
d. CCl3COOH |
Solution4: The electronegativity of chlorine pulls electron density away from the proton making it more acidic. The acid with the greatest number of chlorine is the most acidic. The weakest conjugate base is derived from the strongest conjugate acid.
5. Which mixture of products will be produced in
significant amounts upon mixing of their conjugates? In decreasing strength their relative acidities are: ![]() , H3PO4, HCN, H2O.
, H3PO4, HCN, H2O.
|
a. H3PO4 + CN- |
c. HCN + |
|
b. |
d. HCN + OH- |
Solution5:
Since these are products, the conjugate acid (of the base listed) and
base (of the acid listed) must be stronger than the acid and base found in the
products. HCN is a weaker than H3PO4
and CN- is a stronger base than ![]() .
.
6. Which of the acids listed below is the strongest?
|
a. H2S, Ka = 9.10x10-8 |
c. |
|
b. HBrO, Ka = 2.0x10-9 |
d. HClO, Ka = 3.0x10-8 |
Solution6: The acid with the largest equilibrium constant is the strongest.
7. The pH of a solution of KOH was 10.402. What was the concentration of KOH?
|
a. 1.09x10-4 M |
c. 5.81x10-4 M |
|
b. 2.52x10-4 M |
d. 2.26x10-4 M |
Solution7: The concentration of KOH will equal the [OH-]
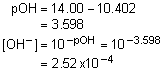
8. Which of the compounds below will not produce a neutral solution when dissolved into water?
|
a. CaBr2 |
c. LiBr |
|
b. Sr(OCl)2 |
d. Sr(ClO4)2 |
Solution8: Look for anions or cations that are not derived from a strong acid or base. OCl- is the conjugate base of HOCl a weak acid. This is thus the salt of a weak acid. The solution will be basic.
9. A solution of F- had a pH of 8.19. What was the [OH-]?
|
a. 2.0x10-6 M |
c. 1.6x10-6 M |
|
b. 8.19 M |
d. 6.4x10-9 M |
Solution9: Determine the pOH and then the hydroxide concentration. We know that answers b and d aren’t reasonable. We need to do the calculations to determine between a and c.
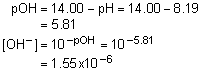
10. Which solution below would have the greatest buffering capacity? Each solution was prepared from a weak base and the salt of its conjugate.
|
a. 0.093 M C5H5N and 0.093 M C5H6NNO3, Kb = 1.4x10-9 |
|
b. 0.055 M C5H5N and 0.055 M C5H6NF, Kb = 1.4x10-9 |
|
c. 0.132 M (CH3)2NH and 0.132 M (CH3)2NH2Cl, Kb = 5.4x10-4 |
|
d. 0.089 M (CH3)2NH and 0.089 M (CH3)2NH2Br, Kb = 5.4x10-4 |
Solution10: The highest buffering capacity will be found with solutions of highest concentration and largest volume! This problem is focussing on the concentrations of the buffer components.
11. Calculate the pOH of 119.1 mL of a buffer initially consisting of 0.1327 M C2H5NH2 and 0.1089 M C2H5NH3NO3 after addition of 0.0022 mol HCl. Assume no volume change occurs after addition of the acid. Kb (C2H5NH2) = 5.6x10-4.
|
a. 10.7 |
c. 3.0 |
|
b. 3.3 |
d. 3.2 |
Solution11: Determine the moles of conjugate base and acid after addition of HCl and then determine the pOH.
12. What is the [OH-] of 0.158 M methylamine? CH3NH2
+ H2O « ![]() + OH- Kb = 3.70x10-4
+ OH- Kb = 3.70x10-4
|
a. 2.45x10-12 |
c. 7.47x10-3 |
|
b. 4.08x10-3 |
d. 1.34x10-12 |
Solution12: This is a weak acid; assume that the [OH-] from the base is greater than from water to get:
![]()
Improve using the method of successive approximations.
![]()
13. What is the equilibrium [OH-] in 0.1413 M H2CO3? H2CO3 + H2O «
H3O+ + ![]() Ka =
4.30x10-7
Ka =
4.30x10-7
|
a. 7.07x10-14 M |
c. 4.06x10-11 M |
|
b. 2.46x10-4 M |
d. 6.84x10-6 M |
Solution13: This is a weak acid. Determine the hydronium ion concentration, and then the hydroxide concentration. We should not that the hydroxide concentration will be relatively small. There is only one answer that fits the concentration level we would expect (answer c).
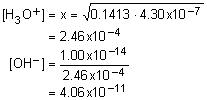
14. A solution consisting of
3.905x10-3 M ![]() was found to be
79.456% ionized. What is the Ka?
was found to be
79.456% ionized. What is the Ka?
![]() + H2O «
+ H2O «
![]() + H3O+
+ H3O+
|
a. 1.01x10-2 |
c. 7.95x10-1 |
|
b. 1.20x10-2 |
d. 1.51x10-2 |
Solution14: Determine the sulfate and hydronium ion concentrations. Substitute into equilibrium expression to determine the equilibrium constant.
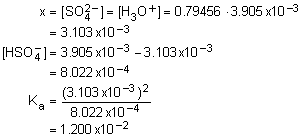
15. What is the pOH of 0.113 M HOC2H4NH3NO3? Kb for HOC2H4NH2 is 3.20x10-5.
|
a. 8.8 |
c. 5.2 |
|
b. 12.0 |
d. 10.3 |
Solution15: This is the salt of a weak base, which is a weak acid. Determine Ka, pH and finally pOH.
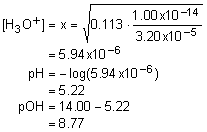
16. When 25.00 mL of a 0.100 M solution of a weak acid was titrated by 0.100 M NaOH, the titration curve below resulted. What is the Ka of this weak acid?
|
a. 7.88x10-7 |
|
b. 1.57x10-8 |
|
c. 3.96x10-5 |
|
d. 5.60x10-11 |
Solution16: The end point is after 25.00 mL of base; after 12.5 mL the pH = pKa = 7.8. Ka = 10-7.8 = 1.58x10-8