Chemistry 212 - Examination 2 |
Name: |
July 21, 1997 |
|
Answers:
1. b |
6. c |
11. a |
16. c |
2. b |
7. b |
12. b |
|
3. a |
8. c |
13. b |
|
4. d |
9. d |
14. c |
|
5. a |
10. d |
15. a |
Instructions: Read the whole examination prior to starting to work on individual problems. Work the problems that you find easiest first. Budget your time!
Solutions:
1. Determine Qc for the reaction below if the pressure decreased by exactly a factor of 1.500
A(g) + B(g)
« 2C(g) + D(g) Kc = 15.33 M2a. 2.25 M2 |
c. 6.81 M2 |
b. 10.22 M2 |
d. 34.49 M2 |
Solution:
When the pressure decreases the volume increases by that same factor. This means that the concentration of each substance decreases by the amount of the pressure decrease compared to the equilibrium pressure.
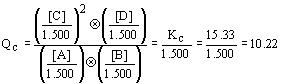
2. Given the equilibrium SO2Cl2(g)
« SO2(g) + Cl2(g) D H = +67 kJ the concentration of SO2 at equilibrium will be increased bya. adding Cl2. |
c. decreasing the volume. |
b. increasing the temperature. |
d. removing SO2Cl2. |
Solution:
When you have an endothermic reaction the reaction will shift to products when the temperature increases.
3. All of the following would be expected to act as Lewis bases except
a. |
c. |
b. |
d. |
Solution:
Lewis base is an electron donor! Draw the Lewis dot structure of each. The only one not having any lone pairs of electrons is answer a.
4. Based upon the list of acids below, the strongest acid is
| CH3COOH | Ka = 1.7x10- 5 | C6H6COOH | Ka = 6.3x10- 5 | |
| HCN | Ka = 4.9x10- 10 | H2O2 | Ka = 1.8x10- 12 |
| a. CH3COOH | c.H2O2 |
b. HCN |
d. C6H6COOH |
Solution:
The largest Ka for this is 6.3x10
- 5; this is the strongest acid.5. Milk of magnesia has a pH of 10.40. What is its [OH
- ]?a. 2.5x10- 4M |
c. 4.0x10 - 11M |
b. 1.0x10 - 7M |
d. 1.0x10 - 4M |
Solution:
![]()
6. Carbon disulfide and chlorine react according to the following equation:
CS2(g) + 3Cl2(g)
« S2Cl2(g) + CCl4(g)When 1.00 mol of CS2 and 3.00 mol of Cl2 are placed in a 2.00-L container and allowed to come to equilibrium, the mixture is found to contain 0.250 mol of CCl4. What is the amount of Cl2 at equilibrium?
a. 0.75 mol |
c. 2.25 mol |
b. 2.75 mol |
d. 0.25 mol |
Solution:
Set up your equilibrium table realizing that the amount of chlorine produced is equal to the x that we have been using.
CS2(g) + |
3Cl2(g) « |
S2Cl2(g) + |
CCl4(g) |
||
Initial |
1.00 |
3.00 |
0 |
0 |
|
Change |
- 0.250 |
- 0.750 |
+0.250 |
0.250 |
|
Equilibrium |
0.750 |
2.250 |
0.250 |
0.250 |
7. Consider the reaction 2A + B
« C + 3D. If, at equilibrium, we find that the molar concentrations of the individual species are A =1, B = 4, C = 8, and D = 2, what will the equilibrium constant be?a. 1/16 |
c. 6 |
b. 16 |
d. 4 |
Solution:
![]()
8. For the reaction NO + 1/2O2
« NO2, at 750°C the equilibrium constant Kp is equal to 1.0. Kc equalsa. 1.0 |
c. Kp(RT)1/2 |
b. Kp(RT)3/2 |
d. Kp(RT) - 1/2 |
Solution:
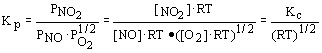
9. For the reaction system
CoO(s) + H2(g)
« Co(s) + H2O(g)at 550°C, K = 67. The equilibrium constant expression is
a. |
c. |
b. |
d. |
Solution:
Do not include solids in equilibrium expression; everything else should be.
10. For the reaction N2(g) + 3H2(g)
« 2NH3(g), the equilibrium constant Kc at 500°C is 6.00x10- 2. The value of Kc for 2NH3(g) « N2(g) + 3H2(g) would bea. - 16.7 |
c. 4.08 |
b. 6.00x10 - 2 |
d. 16.7 |
Solution:
![]()
11. A 0.10 M aqueous solution of an acid HX has a pH of 5.0. What is the value of Ka for HX?
a. 1.0x10- 9 |
c. 1.0x10 - 6 |
b. 1.0x10 - 5 |
d. 1.0x10 - 1 |
Solution:
Solve this using the standard method used for weak acids.
![]()
12. What is the concentration of H+ in a solution labeled 0.075 M HCN? (Ka = 4.8x10
- 10).a. 3.6x10- 11M |
c. 6.4x10 - 9M |
b. 6.0x10 - 6M |
d. 3.0x10 - 5M |
Solution:
We solve this using the standard method involving a weak acid in an aqueous solution. Remember that we can often assume that the extent of dissociation is small compared to 0.075 M.
![]()
13. The pH of a 0.100 M sodium acetate solution (Ka (acetic acid) = 1.75x10
- 5) is| a. 5.76 | c. 6.63 |
| b. 8.88 | d. 2.88 |
Solution:
This is the salt of a weak acid which is of course a weak base; we expect the pH to be slightly basic. Determine Kb and use it with standard equilibrium for the salt of a weak base to determine the pOH; then subtract from 14 to get the pH. Note that since there was only one answer that was basic you did not have to do any calculations at all to determine the correct answer from the ones given.
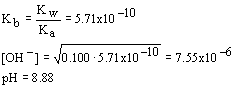
14. A 25.00 mL aliquot of 0.125 M of NH3 (Kb = 1.75x10
- 5) was titrated with 0.115 M HCl; determine the pH after addition of 13.32 mL of acid.| a. 8.80 | c. 9.26 |
| b. 0.94 | d. 11.17 |
Strategy:
This is the titration of a weak base with a strong acid; the moles of base consumed by the acid will equal the mole of acid added. Determine mole of base left and mole of conjugate acid formed; determine pOH and then pH.
Solution:
| 1. Mole of acid added = mol BH+ formed: | nBH = na = Ma*Va = 0.1332· 0.115 mol HCl = 1.533x10- 3 |
| 2. Original # mol base | nb,o = Mb,o*Vb,o =0.025· 0.125 = 3.125x10- 3 |
| 3. Mol base remaining: | nb,r = nb,o -na = 3.125x10- 3 - 1.533x10- 3 = 1.592x10- 3 |
| 4. Conc. of base remaining: | |
| 5. Conc. of BH+ formed | |
| 6. Hydroxide concentration and then pH: | |
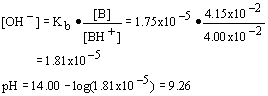 |
15. For the titration in problem 14, what would be the pH at the equivalence point.
| a. 5.23 | c. 5.07 |
| b. 8.77 | d. 8.92 |
Strategy:
At the equivalence point for the titration of a weak base you have generated the salt of a weak base which is a weak acid; determine the concentration of the weak acid and its pKb; then calculate the pH by standard methods.
Solution:
| 1. Volume of strong acid added: | |
| 2. Molarity of acid: | |
| 3. Hydrogen ion concentration and pH: | 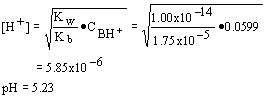 |
16. Let's say that we start out with a buffer at a pH of 6.50 which was made from a weak acid with a pKa of 6.50. Then some base is added to the solution and the pH increases to 6.70. Determine the final ratio of concentrations of conjugate base to conjugate acid ([A
- /[HA]).| a. 0.500 | c. 1.58 |
| b. 3.33 | d. 0.631 |
Solution:
| When we know pH and pKa, we can calculate the ratio of the two concentrations using the standard buffer equation. | 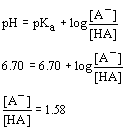 |