|
Chemistry 422 Exam 2 |
Name: |
|
|
October 26, 2001 |
please print |
Instructions: Read the whole exam before starting to work on individual problems; work those that you find the easiest first. Budget you time!
1. a. The time dependant amplitude of two waves are given by the following general equation: y = 5*sin(2
pnt + f). The amplitude at t = 0 and frequency of the first wave were 0.00 and 20 s- 1 while for the second one at t = 0 the amplitude and frequency were 5 and 22 s- 1. What is the amplitude of the composite wave of these two after 1 second?b. Draw a diagram of a Czerny-Turner monochromator and explain the principles of its operation.
c. What length of mirror drive in a Michelson interferometer is required to produce a resolution sufficient to separate infrared peaks at 20.11
mm and 20.12 mm?d. Draw the major components of an fluorescence spectrophotometer. Describe in detail the principles of its operation.
2. a. Draw a diagram of a hollow catho de tube and explain the principles of its operation.
b. The wavelengths for the 3s ® 3p and 3p ® 4s transitions of sodium are 589.6 nm and 1138.3 nm, respectively. What will be the ratio of atoms in the 4s state relative to the 3s state in an inductively couple plasma source that has a temperature of 9000 K?
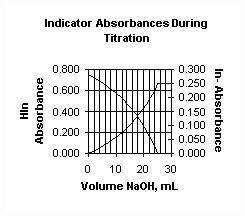
3. A 25.00 mL of 0.100 M weak acid was titrated with 0.100 M NaOH; an indicator, 5.00x10-4 M, was employed to help determine the endpoint colorimetrically by monitoring the absorbance at two wavelengths. What is the Ka for the weak acid, if Ka = 7.94x10-6 for the indicator? Assume no volume corrections are needed in solving the problem.
4. a. The wavelength of vibration for the N – H bond is about 3.00 mm. What is the approximate wavenumber expected for the N – D stretch?
b. Draw a potential energy diagram for the harmonic oscillator and the anharmonic oscillator and discuss when the harmonic oscillator can be used.
5. a. Explain what is meant by a singlet, doublet and triplet state in spectroscopy.
b. Show that the fluorescence intensity is proportional to the concentration of the substance fluorescing. Make sure to explain when the equation is applicable.
c. Explain why fluorescence spectroscopy is usually more sensitive than absorption spectroscopy.