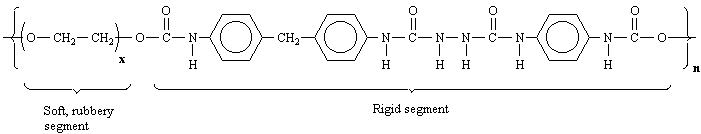
Notice that because Spandex is a polymer, its macromolecular structure is made up of repeating units (mers) denoted by the x and n next to the parentheses in the structure. Each Spandex fiber will differ somewhat in length and composition depending on the exact values of x and n.
Expanded Structure:
Draw in the missing hydrogens and non-bonded electrons in the abbreviated
structure of Spandex.
Functional Groups:
Spandex contains some composite functional groups not mentioned in the
early chapters of your text. First, circle each of the simple functional
groups in the abbreviated structure of Spandex. A urethane is a
composite functional group that is part ester and part amide. Circle the
urethane functional group(s). You should know the structure of urea.
Circle the substituted urea functional group(s).
Hybridization and Geometry:
For each of the atoms in the abbreviated Spandex structure, state its
hybridization and geometry. (Remember the "amide" rule -- the
N of amides is sp2 hydbridized.)
Functional groups that have resonance contributors (and thus double-bond character) will have higher barriers to rotation. Find the urethane and urea derivative functional groups in the structure above and show how the carbonyl group and the adjacent heteroatom can be drawn as another resonance structure.
Conformational Analysis:
With respect to the resonance structures you drew, state which have higher
or lower energy barries to rotation. Explain why there is a barrier to
rotation for these groups.
Here is a small Spandex molecule in one possible conformation.
Dependence of Physical Properties on Structure:
With respect to your answers above, explain why one segment of Spandex
is "soft and rubbery" and the other segment is "rigid."