Questions for Chapter 4
1. a) Draw a structure for isofluorane, also known as 1-chloro-2,2,2-trifluoroethyl difluoromethyl ether. [Answers]
b) Expand the structure CH3CH2C(CH3)Br(CH2)2CH(CH3)2. Explain why it is named 5-bromo-2,5-dimethylheptane and not 3-bromo-3,6-dimethylheptane.
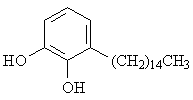
2. The structure shown below is a hydrocarbon derivative of the diol commonly called "catechol". The compound is a primary component of poison ivy's urushiol oil. Name the hydrocarbon substituent on the aromatic ring. [Answer]
3. A question in the Supplement asked you to draw all constitutional isomers of the cycloalkanes C4-C6. As an example, a few of the isomers of cyclo-C7H14 were shown. There are a total of 29 (not 27 - thanks, Lisa) cycloalkane isomers with this molecular formula (disregarding stereochemistry). Draw them all.
[Answer]
4. This journal article was titled "Axial/Equatorial Stability Reversal in all-trans-Polyalkylcyclohexanes" [JACS 1990,112, 9300.] Answer the questions concerning aspects of the article.
A. The research concerned all-trans-hexaisopropylcyclohexane, which was prepared from catalytic hydrogenation of dodecamethyl[6]radialene, shown below.
- Account for the "dodecamethyl" part of the radialene name.
- Draw an expanded structure for the isopropyl group and then draw the structure for hexaisopropylcyclohexane.
- Write a chemical equation that shows the hydrogenation reaction of the radialene to hexaisopropylcyclohexane.
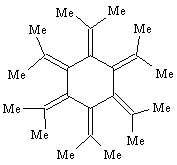
B. The product hexaisopropylcyclohexane was found to have all six isopropyl groups in the axial instead of the expected equatorial positions. According to the X-ray structure, all the isopropyl groups direct their methine hydrogens toward the center of the cyclohexane ring.
- Draw a chair conformation for the product showing the stereochemistry of the substituent groups.
- Identify the methine hydrogen in the isopropyl group.
[Answers] This is similar to question 4.44 in Solomons 12th ed.
The authors state that it is difficult to pinpoint the exact cause for the stability reversal phenomenon. It is probably due to destabilization of the all-equatorial form rather than to stabilization of the all-axial form.
5. This question is from the Supplement. Show how you could synthesize the compound below.
CH3CD=CDCH3 (cis) from 2-butyne[Answer]
6. Here are some isomers of heptane. On the basis of their structures, predict their stability order (if they are of comparable stablility, state so).
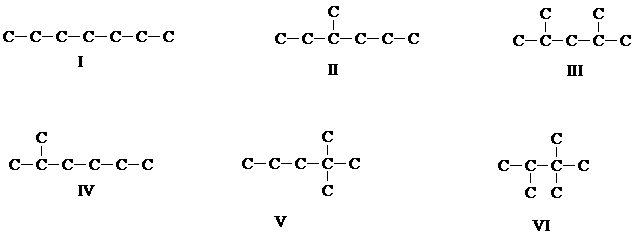
[Answer]
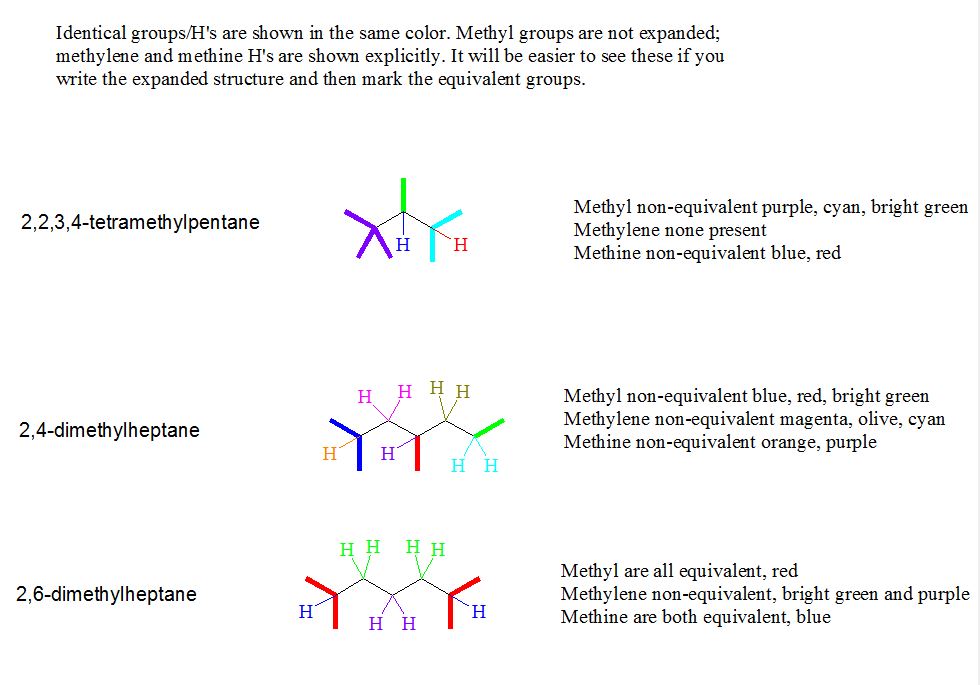
7. The Supplement problem asked you to identify equivalent and non-equivalent carbons and hydrogens in some of the nonane isomers. Try these: 2,2,3,4-tetramethylpentane; 2,4-dimethylheptane; 2,6-dimethylheptane.
[Answer]
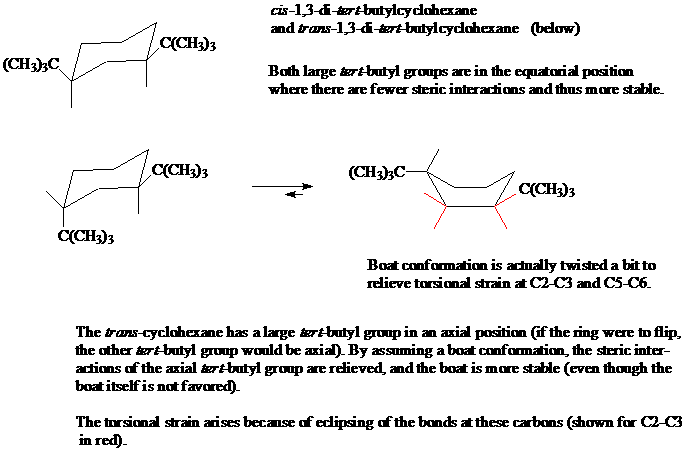
8. Here is an example of a cyclohexane derivative where the boat conformation is more stable than the chair conformation.
a) Name both of these disubstituted cyclohexanes, making sure to include the stereochemical descriptors of cis or trans.
b) Explain why the conformation at top left is the only conformation present (no "flipped" conformation is present in a mixture).
c) Using a molecular model of the lower left cyclohexane, show why the chair-to-boat conversion results in a more stable conformation, even though unsubstituted cyclohexane does not exist in the boat conformation.
d) Draw in all the bonds in the chair and boat conformations. Explain what is meant by the "torsional strain at C2-C3 and C5-C6" in the illustration below.
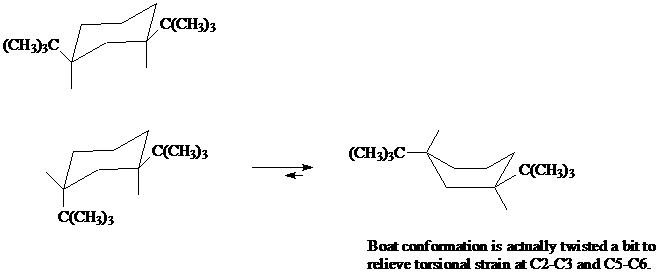 [Answer]
[Answer]
Here is a site with nomenclature problems of various types. All have answers.
https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/questions/Nomencl/nomencl.htm
Try the problems from the MIT Open Course site Problem Set 2 (#5a, c, 6, 7, 8, 9); 3(#5)
There are answers also.

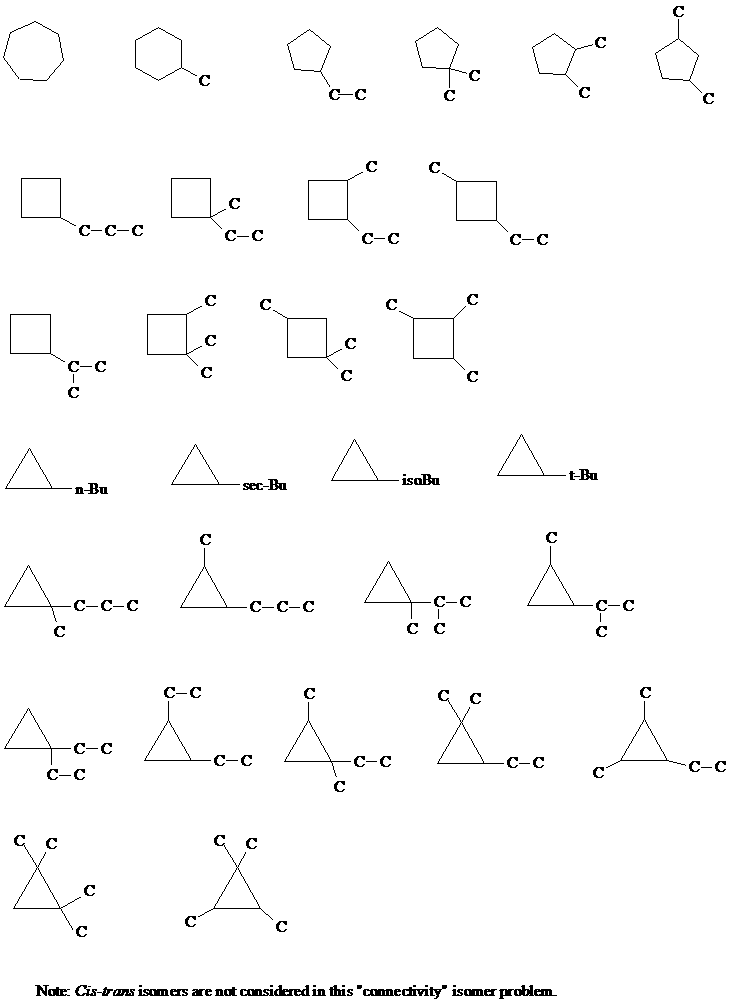
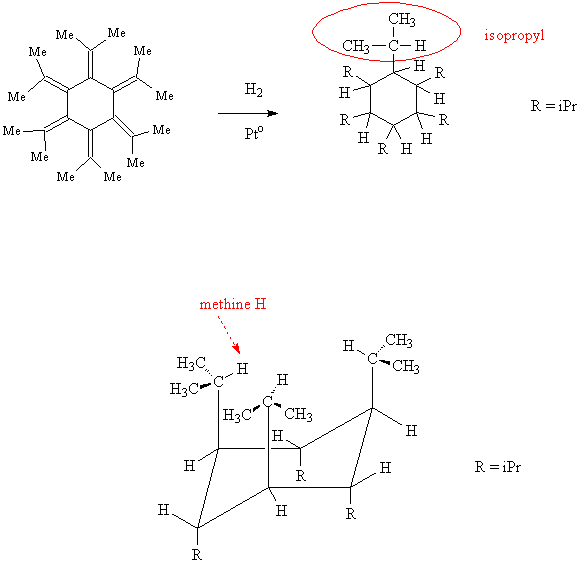 (There are also methine H's attached to every C of the cyclohexane.)
(There are also methine H's attached to every C of the cyclohexane.)