Questions for Chapter 5
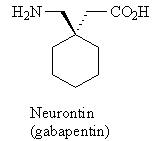
1. Is Neurontin chiral or achiral? (Neurontin is Pfizer's anticonvulsive drug -- $1.48 billion in sales for the first half of 2004 before the introduction of the generic drug, gabapentin.)
[Answer]
2. This headline
from a recent edition of "Chemical & Engineering News":
Sulfanyl alcohols are culprits in smelly armpits. Research groups
at two Swiss fragrance and flavor companies have identified the compounds,
the major component of which is shown. 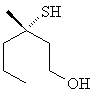
What is the specification of configuration, R or S? [Answer]
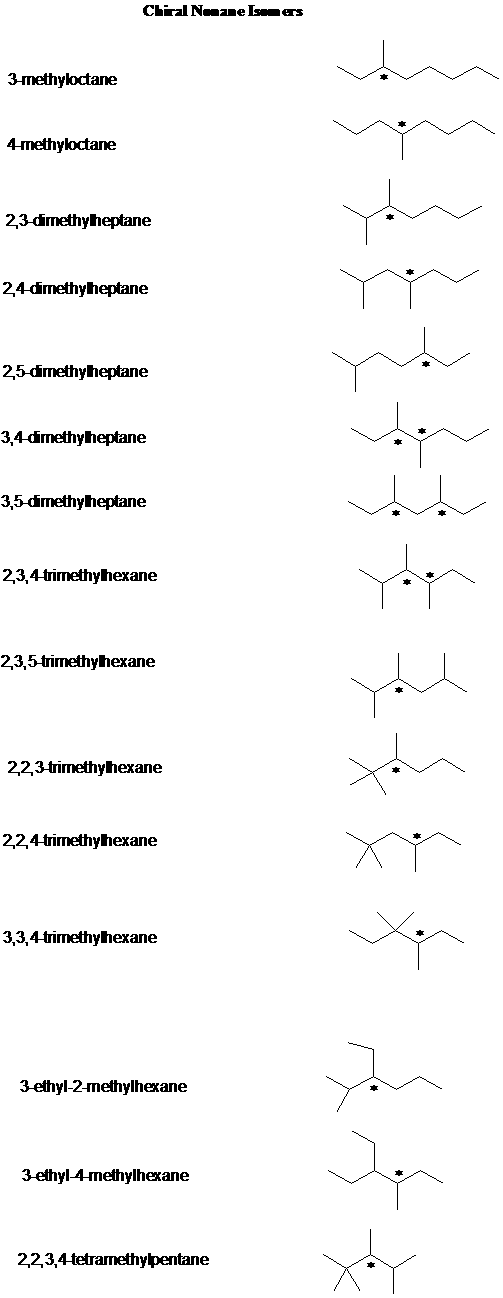
3. A question in the Supplement asked you to identify the chirality centers (if any) in each of the nonane isomers. Here is the answer.
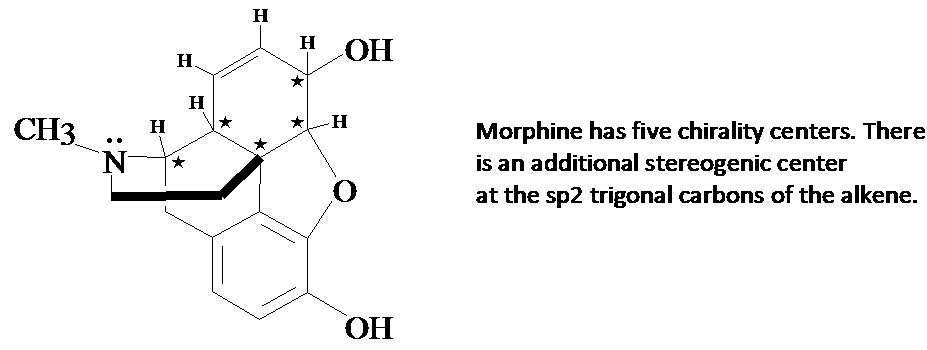
4. A question in the Supplement asked how many chirality centers there were in the morphine molecule. Here is the answer.
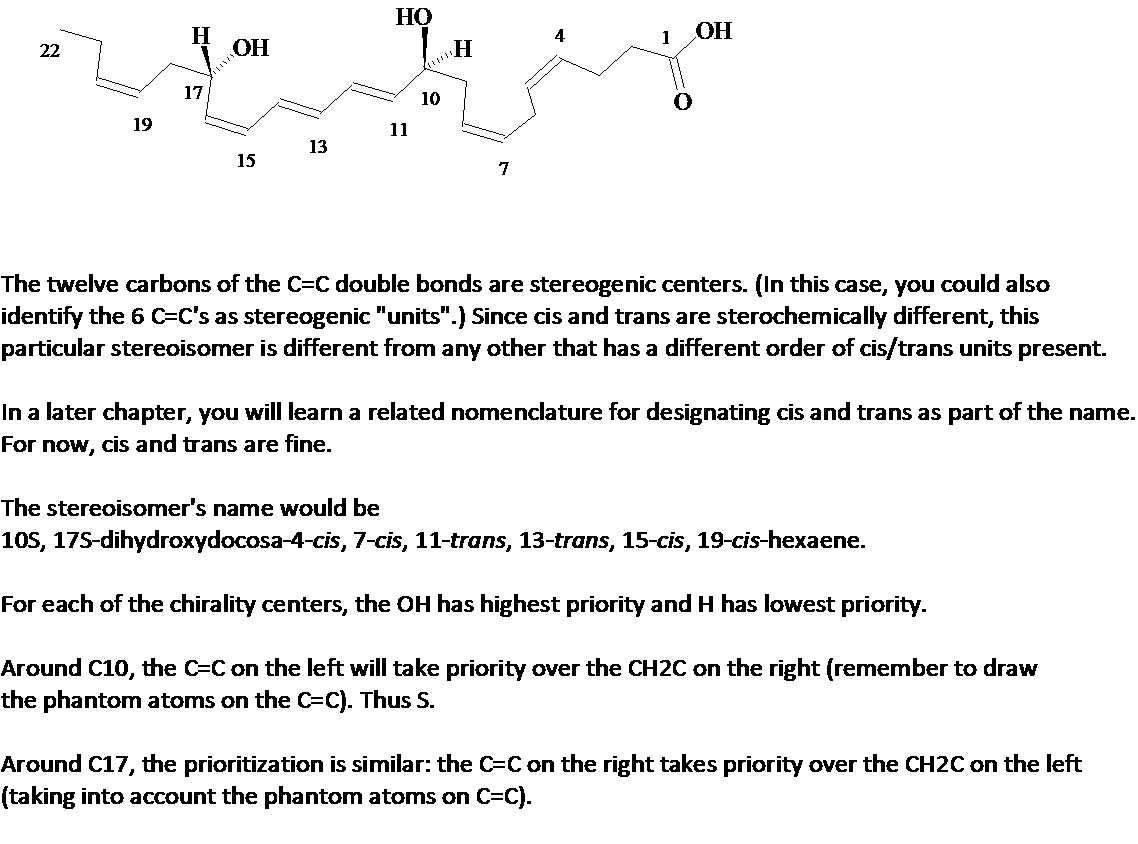
5.The protectin D1 stereoisomer 10S,17S-dihydroxydocosahexaenoic acid blocks replication of the flu virus. In addition to the two chirality centers shown, there are other stereogenic centers in the molecule. How many, and which are they? A full description of this stereoisomer would include the specification of configuration of these stereogenic centers. Start the parent chain numbering with the carbon of the carboxylic acid group, and specify these cis/trans configurations. Rationalize the designation of both chirality centers as S. Answer.
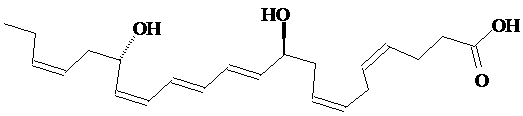
Try the problems from the MIT Open Course site Problem Set 2 (#11, 12, 13);1 (#2)
There are answers also.